The Katukojvala group main focus is to develop new catalytic activations in the metal-carbene and radical chemistry. The group also engaged in designing novel catalytic processes with the emphasis on atom economy and selectivity which would benefit both academia and pharmaceutical industry. The group works at the interface of Organometallic/Organic Chemistry to design new reactions/catalysts.
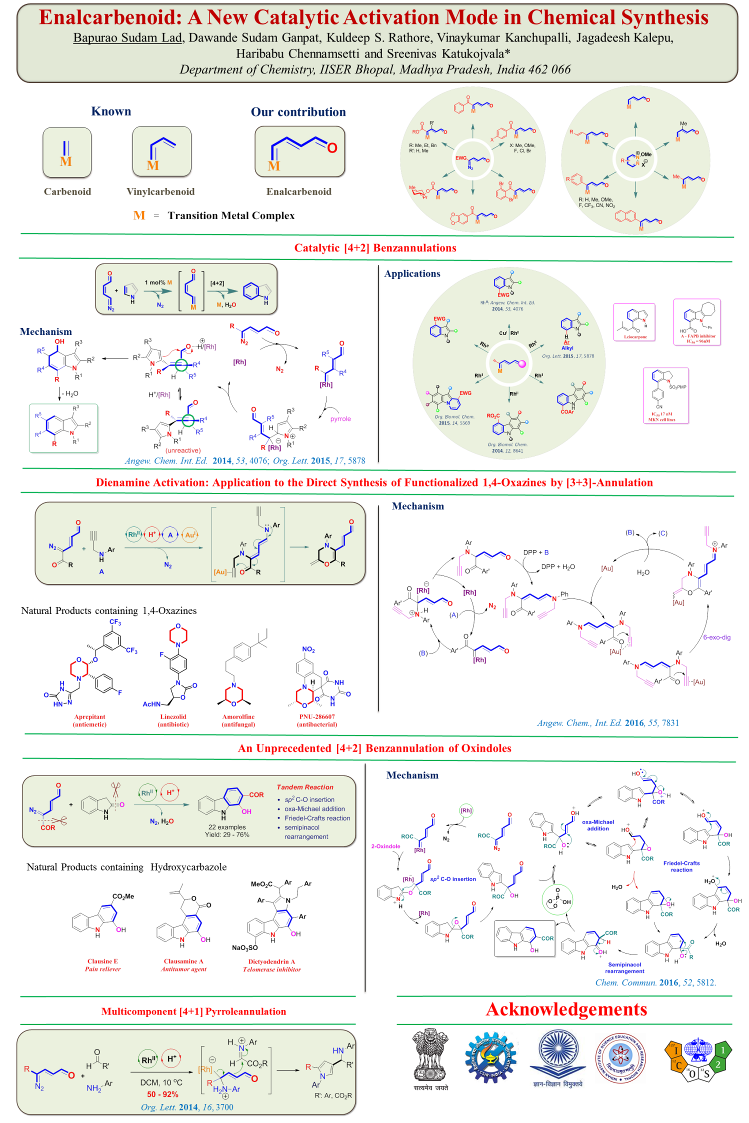
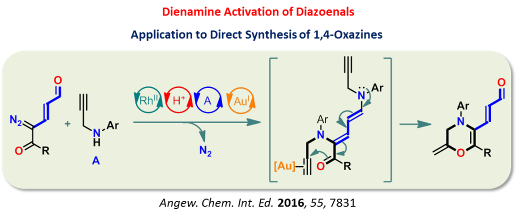
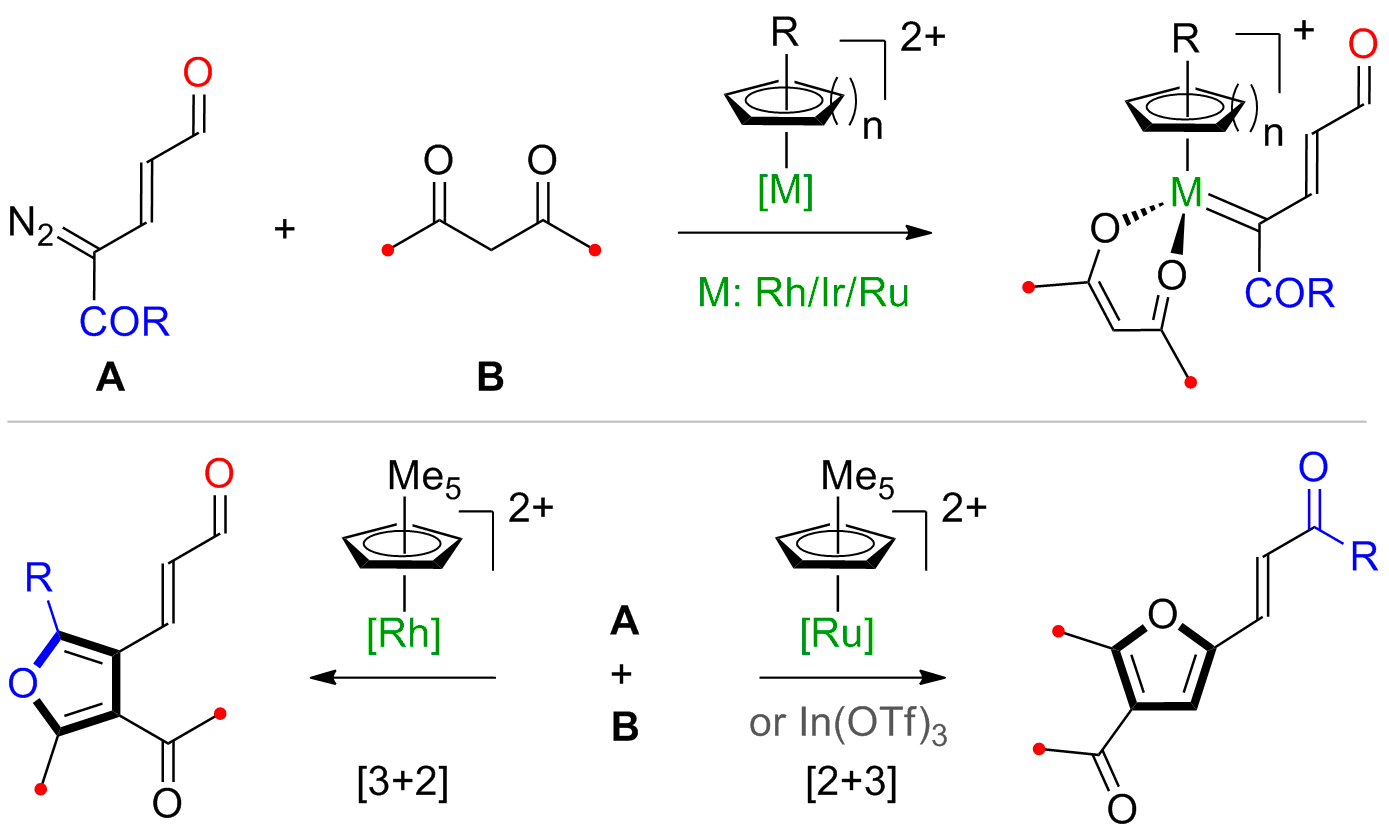
Recently, our group introduced a conceptually new class of hybrid functional groups diazoenals. These hybrid functional groups are the rich source of new activation modes that would offer
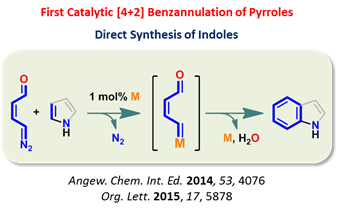
unprecedented reactivity through the controlled reactivity of diazo and enal functionalities integrated through the efficient π-conjugation. Chemoselective LUMO activation of diazoenal by
transition-metal catalysis resulted in a highly electrophilic enalcarbenoid (Scheme 1). In addition, a cascade transition-metal catalysis and aminocatalysis offered HOMO activation of
diazoenal leading to valuable γ-functionalized dienamines (Scheme 2). These two activation modes led to the discovery of distinct new catalytic reactions for the one-step construction of
valuable nitrogen and oxygen heterocycles.
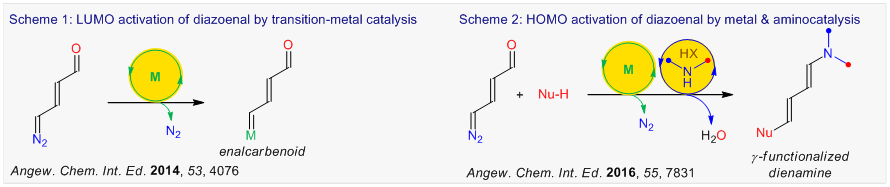
Our group also introduced diverse pyridazinium salts as the safe, air stable, benchtop precursors for the diazoenals and enalcarbenoids (Scheme 3). Pyridazinium salts can be prepared in
multigram scale and stored at ambient temperature.
Org. Lett. 2015, 17, 5878
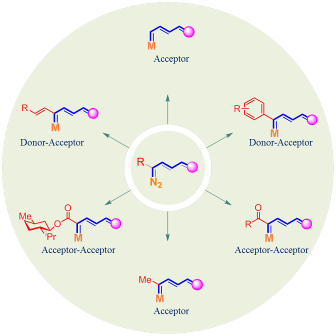
Design of Diverse Enalcarbenoids
Synthetic Applications of Enalcarbenoids
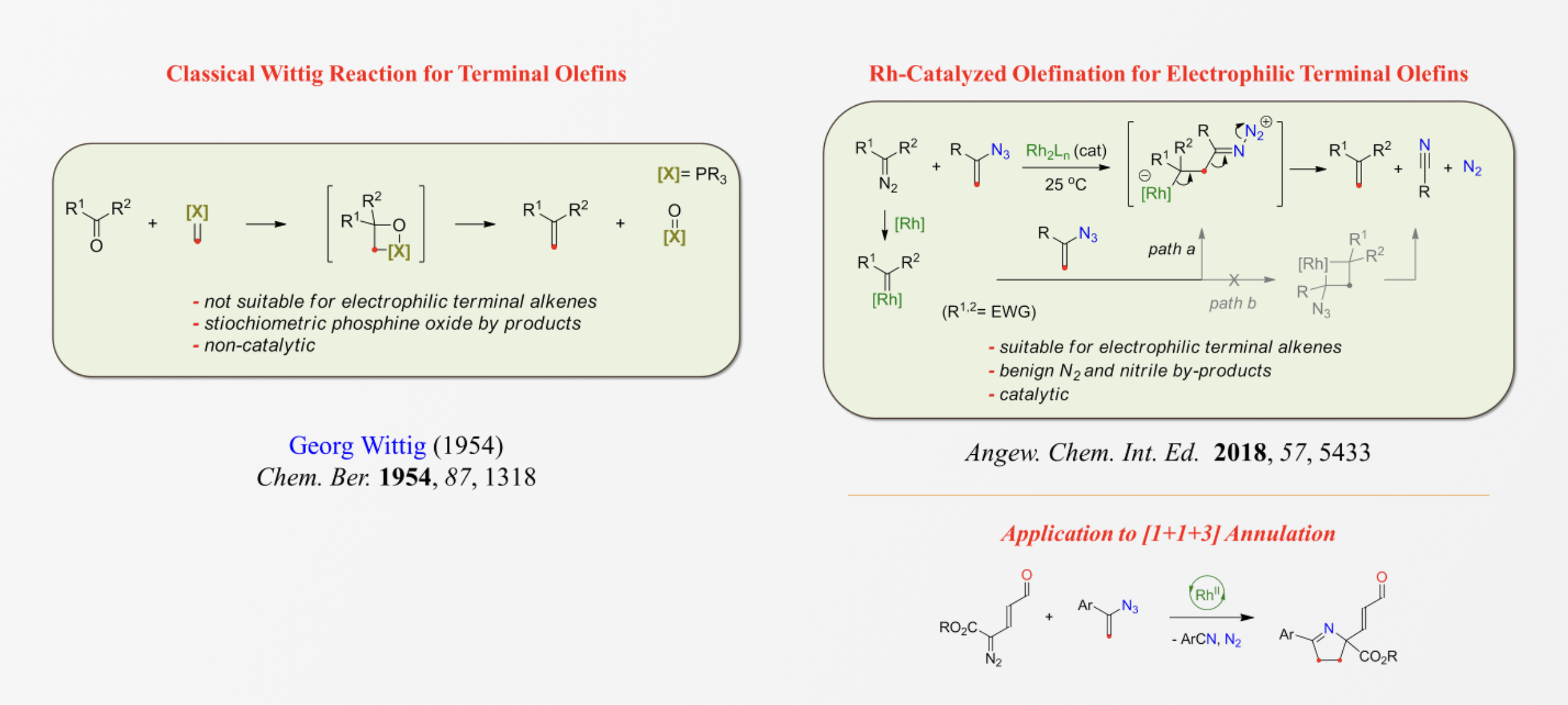
ACS Catal. 2018, 8, 11807