Research Interest:
Experimental Condensed Matter Physics: Electronic structure of condensed matter systems having novel properties such as metal insulator transitions, high Tc superconductivity, unusual magnetism, non Fermi liquid behavior, charge and spin density wave state, etc. using high energy and angle resolved photoelectron spectroscopy.
Photoemission spectroscopy
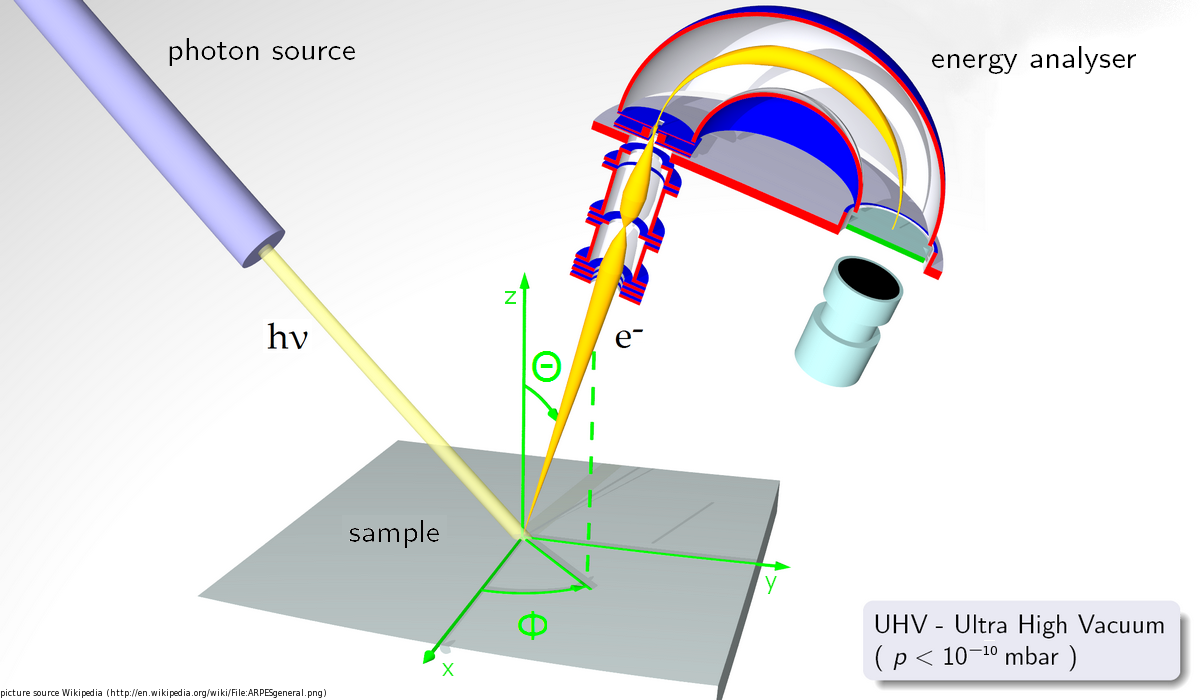
Principle: Photoemission spectroscopy (PES), also known as photoelectron spectroscopy, is a direct probe to study the electronic structure. It is based on the principle of photoelectric effect, where a material is irradiated with the photons. If the energy of the photons is higher than the work function (minimum energy required to emit an electron), electrons are knocked out of the sample. The excitation of the electrons due to these photons follows energy conservation rule, hν = Ekin + Ebin + φ. Here, hν is the energy of the incident photon, Ebin and Ekin are the binding energy and the kinetic energy of the photoexcited electrons, and φ is known as work function. Thus, in this process, one can experimentally determine the binding energy of the electron by measuring the kinetic energy of the photo-emitted electrons.
Depending on the energy of the photons used in this process, the technique is termed as x-ray photoelectron spectroscopy (XPS; hν > 1000 eV), soft x-ray photoemlectron spectroscopy (SXPS; 100 < hν < 1000 eV), ultra violet photoelectron spectroscopy (UPS; 10 < hν < 100 eV) etc. The standard laboratory sources for the XPS are Mg Kα (1253.3 eV) and Al Kα (1486.6 eV) and for UPS, the most commonly used photon source is ultraviolet Helium lamp which produces He I (21.2 eV) and He II (40.8 eV) photons. Using the synchrotron radiation source, many other photons can be obtained ranging from ultraviolet to hard x-rays, and the technique is called as synchrotron radiation photoelectron spectroscopy (SRPES).
XPS was developed by Kai Siegbahn starting in 1957 and is used to study the energy levels of atomic core electrons, primarily in solids. Siegbahn referred to the technique as Electron Spectroscopy for Chemical Analysis (ESCA). Siegbahn was awarded the Nobel Prize in 1981 for his contribution to the development of high-resolution electron spectroscopy.
Methodology: In this technique one determines the kinetic energy of the photoelectrons using a high sensitivity electron energy analyzer and electron detector, which provides information about the binding energy of the associated transition. Thus, one can map the total density of states as a function of binding energy, which is essentially the electronic structure of the system under study. Since, the escape depth of the photoexcited electrons is small (15-25 Å in case of XPS and 5-10 Å in case of UPS) it is important to perform these measurements on an extremely clean sample surface. Thus, all the measurements have to be carried out in an ultra high vacuum (UHV) chamber (vacuum ~ 10-11 mbar).
A typical photoemission spectrometer consists of UHV chamber equipped with various laboratory based monochromatic photon sources (UV source and x-ray source etc.), electron energy analyzer with detector, sample manipulator with provisions to perform experiments at low temperatures, various sample preparation/cleaning/characterization options etc.